July 1, 2021
Possible contribution of the DNA repair pathway selection property of RecQ4 to the elucidation of the pathogenesis of the Rothmund-Thomson Syndrome




Key points
RecQ4 was implicated as the causative agent of Rothmund-Thomson syndrome. The authors demonstrated that the N-terminal fragment of RecQ4 was shown to inhibit non-homologous end-joining repair, which is important in DNA double-strand break repair. Further analyses revealed that RecQ4 inhibits the binding of Ku70, an essential protein for non-homologous end-joining repair, to DNA double-strand breaks, and the inhibition is dependent on the enzymatic activity of ATM, a serine/threonine kinase involved in homologous recombination repair. The findings clarify the role of RecQ4 in DNA double-strand break repair and the pathogenesis of the Rothmund-Thomson syndrome. The findings also enhance our molecular understanding of aging and carcinogenesis.
RecQ4 was implicated as the causative agent of Rothmund-Thomson syndrome. The authors demonstrated that the N-terminal fragment of RecQ4 was shown to inhibit non-homologous end-joining repair, which is important in DNA double-strand break repair. Further analyses revealed that RecQ4 inhibits the binding of Ku70, an essential protein for non-homologous end-joining repair, to DNA double-strand breaks, and the inhibition is dependent on the enzymatic activity of ATM, a serine/threonine kinase involved in homologous recombination repair. The findings clarify the role of RecQ4 in DNA double-strand break repair and the pathogenesis of the Rothmund-Thomson syndrome. The findings also enhance our molecular understanding of aging and carcinogenesis.
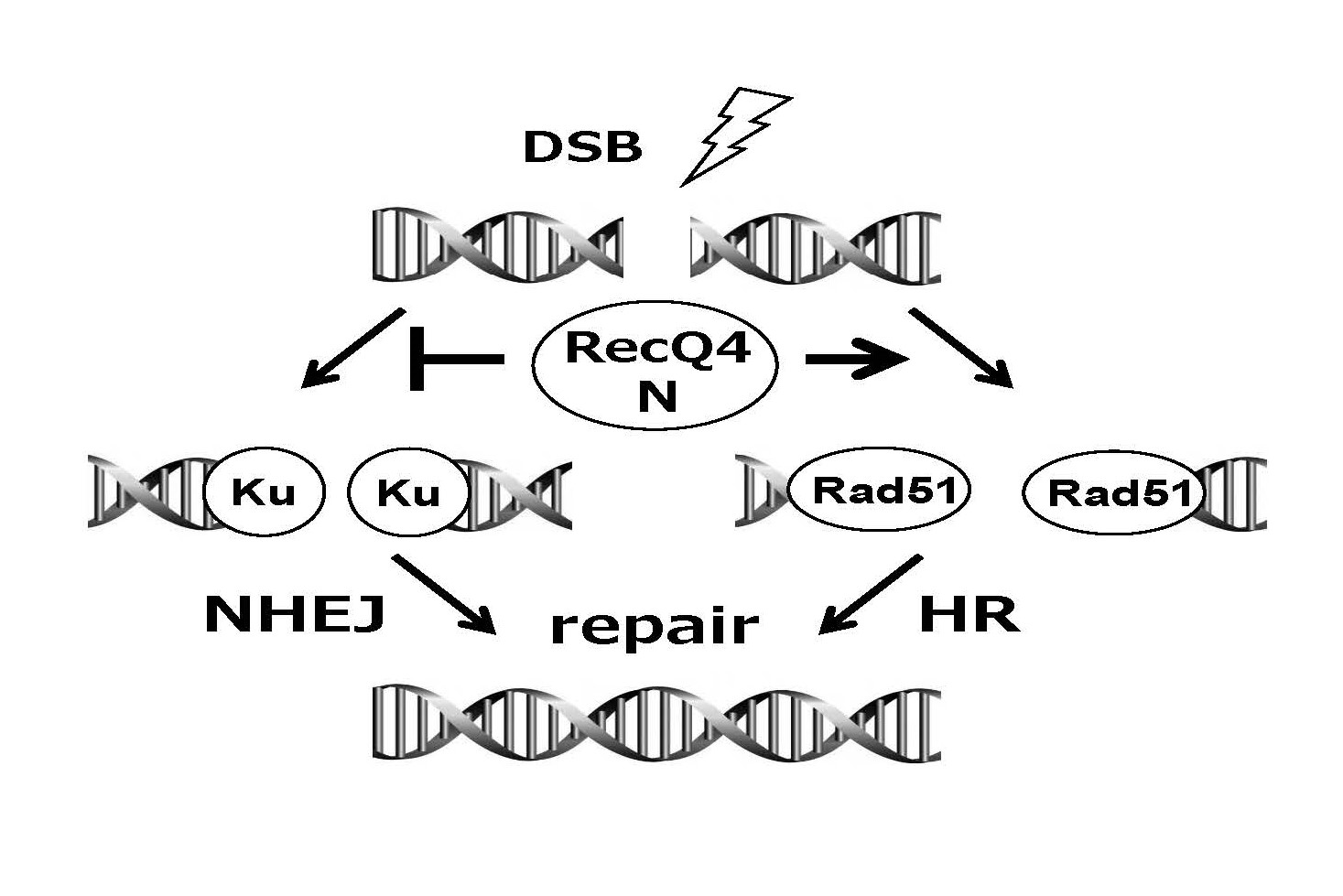

Possible involvement of RecQ4 in the DSB repair pathway choice via the N-terminal region.
N-terminal region of RecQ4 suppresses non-homologous end-joining, and promotes homologous recombination for the repair of DNA-double strand breaks (DSBs). Because the N-terminal region, but not the rest of RecQ4 regions, is thought to be active in the cells from patients of Rohmund-Thomson syndrome, the function of the RecQ4 N-terminal region for the choice of DSB-repair pathway is presumably concerned with the etiology of the hereditary disease.
Summary
DNA double-strand breaks (DSBs) are induced in response to radiation or anticancer drugs. DSBs are the most serious form of DNA damage. Repair of DSBs mainly involves one of two repair pathways: non-homologous end-joining repair and homologous recombination repair. Cells appear to have the ability to select between these repair pathways depending on the situation. The pathway selection mechanism has been studied more intensively in recent years. RecQ4 is mutated in the Rothmund-Thomson syndrome, a disease characterized by premature aging and high risk of carcinogenicity. This protein functions in the DSB repair pathway, but the details of its function are unclear. The research group led by Professor Shusuke Tada and Lecturer Takashi Tsuyama at the Department of Molecular Biology, Faculty of Pharmaceutical Sciences, Toho University, established an experimental system that can reproduce the DSB repair system in Xenopus egg extracts. Experimental results suggested the involvement of RecQ4 in the pathway selection of DSB repair. The results of this study help elucidate the role of RecQ4 in DNA repair and will further our understanding of the molecular underpinnings of the pathogenesis of Rothmund-Thomson syndrome, aging, and cancer.
DNA double-strand breaks (DSBs) are induced in response to radiation or anticancer drugs. DSBs are the most serious form of DNA damage. Repair of DSBs mainly involves one of two repair pathways: non-homologous end-joining repair and homologous recombination repair. Cells appear to have the ability to select between these repair pathways depending on the situation. The pathway selection mechanism has been studied more intensively in recent years. RecQ4 is mutated in the Rothmund-Thomson syndrome, a disease characterized by premature aging and high risk of carcinogenicity. This protein functions in the DSB repair pathway, but the details of its function are unclear. The research group led by Professor Shusuke Tada and Lecturer Takashi Tsuyama at the Department of Molecular Biology, Faculty of Pharmaceutical Sciences, Toho University, established an experimental system that can reproduce the DSB repair system in Xenopus egg extracts. Experimental results suggested the involvement of RecQ4 in the pathway selection of DSB repair. The results of this study help elucidate the role of RecQ4 in DNA repair and will further our understanding of the molecular underpinnings of the pathogenesis of Rothmund-Thomson syndrome, aging, and cancer.
READ MORE RESEARCH NEWS - Pharmaceutical Sciences
ACADEMICS
Undergraduate Programs
– Medicine
– Pharmaceutical Sciences
– Science
– Nursing
– Health Science
Graduate Programs
–Medicine
–Pharmaceutical Sciences
–Science
–Nursing
Undergraduate Programs
– Medicine
– Pharmaceutical Sciences
– Science
– Nursing
– Health Science
Graduate Programs
–Medicine
–Pharmaceutical Sciences
–Science
–Nursing
RESEARCH
– News
– Guidelines & Policies
– Support Offices
– Facilities
– Security Export Control
Non-Degree Programs
– Clinical Elective Program
– International Physician Observership Program

.jpg)
