September 8, 2021
Chiral Crystallization of Optically Inactive Indolyl Sulfonamides Caused Through the Combination of Specific Intermolecular Interactions
Professor Isao Azumaya and Research Associate Ms. Shoko Kikkawa at the Faculty of Pharmaceutical Sciences of Toho University and their research group have discovered that optically inactive indolyl sulfonamides exhibit chiral crystallization.
This yields optically active crystals in a high proportion, depending on the type and combination of specific intermolecular interactions. This finding brings us closer to the nature of chiral crystallization phenomenon and is expected to lead to the development of new synthetic methods for optically active materials, as optically active crystals can be obtained from optically inactive solutions by crystallization alone.

Key points of the presentation
The researchers found that optically inactive indolyl sulfonamides (benzenesulfonamide derivatives of indoleamine) formed optically active crystals at an unusually high proportion compared with other common compounds via the process of chiral crystallization.
The chiral crystallization in indolyl sulfonamides was affected by the specific type of interaction between molecules in the crystal. This was demonstrated by a comprehensive analysis using Mchiral, the ratio of chirality identities of molecules linked by interactions proposed by the authors.
In the crystal, the most dominant interactions between the indolyl sulfonamide molecules are two types of hydrogen bonds: hydrogen bonding interactions between the sulfonamide proton and the sulfonyl group of the sulfonamide, and hydrogen bonding interactions between the NH proton of the indole and the sulfonyl group of the sulfonamide. The high proportion of chiral crystallization is thought to be mainly due to the combination of these two types of hydrogen bonds. Furthermore, among the intermolecular interactions other than those mentioned above, the CH…π interaction was favorable for chiral crystallization, whereas the interaction between the hydrogen bond and π…π was not.
The researchers found that optically inactive indolyl sulfonamides (benzenesulfonamide derivatives of indoleamine) formed optically active crystals at an unusually high proportion compared with other common compounds via the process of chiral crystallization.
The chiral crystallization in indolyl sulfonamides was affected by the specific type of interaction between molecules in the crystal. This was demonstrated by a comprehensive analysis using Mchiral, the ratio of chirality identities of molecules linked by interactions proposed by the authors.
In the crystal, the most dominant interactions between the indolyl sulfonamide molecules are two types of hydrogen bonds: hydrogen bonding interactions between the sulfonamide proton and the sulfonyl group of the sulfonamide, and hydrogen bonding interactions between the NH proton of the indole and the sulfonyl group of the sulfonamide. The high proportion of chiral crystallization is thought to be mainly due to the combination of these two types of hydrogen bonds. Furthermore, among the intermolecular interactions other than those mentioned above, the CH…π interaction was favorable for chiral crystallization, whereas the interaction between the hydrogen bond and π…π was not.
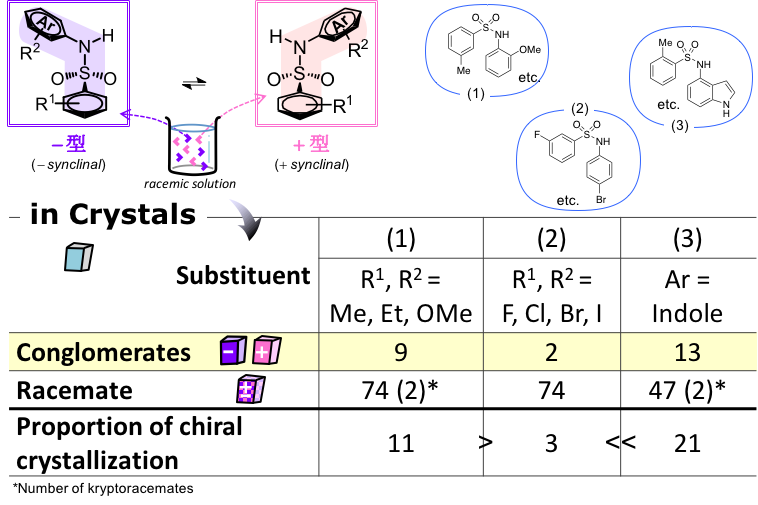
figure 1: Types of functional groups and percentages of compounds that give chiral crystals and racemic crystals
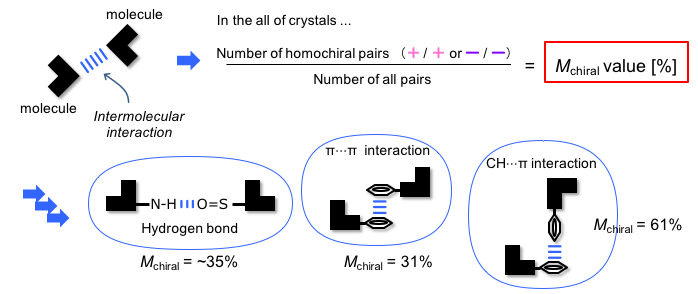
figure 2: Major interactions and the percentage of homochiral associations through these interactions
(Mchiral value)
Journal name:Crystal Growth & Design (July 8, 2021 issue)
Title of paper:High Proportion of Chiral Crystallization of Achiral Indolyl Sulfonamides: Effect of Intermolecular Interactions.
DOI Number:10.1021/acs.cgd.1c00305
Title of paper:High Proportion of Chiral Crystallization of Achiral Indolyl Sulfonamides: Effect of Intermolecular Interactions.
DOI Number:10.1021/acs.cgd.1c00305
READ MORE RESEARCH NEWS - Pharmaceutical Sciences
ACADEMICS
Undergraduate Programs
– Medicine
– Pharmaceutical Sciences
– Science
– Nursing
– Health Science
Graduate Programs
–Medicine
–Pharmaceutical Sciences
–Science
–Nursing
Undergraduate Programs
– Medicine
– Pharmaceutical Sciences
– Science
– Nursing
– Health Science
Graduate Programs
–Medicine
–Pharmaceutical Sciences
–Science
–Nursing
RESEARCH
– News
– Guidelines & Policies
– Support Offices
– Facilities
– Security Export Control
Non-Degree Programs
– Clinical Elective Program
– International Physician Observership Program

.jpg)
