July 5, 2021
A Phase III Clinical Trial on Epidermolysis Bullosa Treatment with Regenerative Medical Products Initiated

Professor and Chairman
Department of Dermatology
Toho University Omori Medical Center
Epidermolysis bullosa is a hereditary disease that causes blister, erosion, and ulcer formation all over the body, especially in areas susceptible to mechanical stimulation; the pathogenesis is linked to a congenital deficiency, or an extreme lack of factors important to the adhesion between the epidermis and dermis, such as type VII collagen. Since it persists throughout life, it has been labelled as an intractable disease by the Ministry of Health, Labor and Welfare.
The treatment under investigation involves the application of a regenerative medical product containing mesenchymal stem cells derived from adipose tissue of a healthy donor to the ulcer site. This is expected to supply the affected areas of skin with various growth factors that promote skin formation from other stem cells, as well as type VII collagen, which is lacking in these patients, to the lesions, thereby promoting ulcer healing and improving skin fragility.

Professor and Chairman
Department of Dermatology
Toho University Omori Medical Center
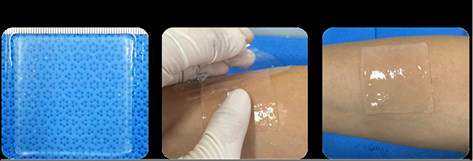
Adipose-derived mesenchymal stem cell sheet product. The colorless, transparent, 5 cm square jelly-like sheet on one side shows the appearance when applied to normal skin.
For questions, contact Prof. Akira Ishiko
Department of Dermatology, Faculty of Medicine, Toho University
at akira.ishiko[@]med.toho-u.ac.jp
Department of Dermatology, Faculty of Medicine, Toho University
at akira.ishiko[@]med.toho-u.ac.jp
READ MORE RESEARCH NEWS - MEDICINE
ACADEMICS
Undergraduate Programs
– Medicine
– Pharmaceutical Sciences
– Science
– Nursing
– Health Science
Graduate Programs
–Medicine
–Pharmaceutical Sciences
–Science
–Nursing
Undergraduate Programs
– Medicine
– Pharmaceutical Sciences
– Science
– Nursing
– Health Science
Graduate Programs
–Medicine
–Pharmaceutical Sciences
–Science
–Nursing
RESEARCH
– News
– Guidelines & Policies
– Support Offices
– Facilities
– Security Export Control
Non-Degree Programs
– Clinical Elective Program
– International Physician Observership Program