August 24, 2022
Role of the Transcriptional Regulator IκBζ in Intestinal Epithelial Cell-Mediated Immunity for Maintenance of Intestinal Microbiota Balance
A study performed by Dr. Soh Yamazaki and Prof. Hiroyasu Nakano of Toho University Faculty of Medicine reported that IκBζ serves as a transcriptional regulator of gene expression in small intestinal epithelial cells and thereby regulates the intestinal microbiota.
Defects in this control mechanism lead to an imbalance of the intestinal microflora, which triggers abnormal activation of immune cells throughout the body and consequently increases the risk of autoimmune diseases that affect various organs. This discovery has improved our understanding of the mechanisms underlying regulation of the intestinal microbiota and opened up novel therapeutic avenues for management of various autoimmune diseases, including inflammatory bowel disease. The results were published online in Mucosal Immunology on August 24, 2022. This research was performed in collaboration with Dr. Yosuke Tsuneoka, Department of Anatomy, Toho University Faculty of Medicine; Dr. Naohiro Inohara, University of Michigan Medical School, The United States; and Prof. Masaki Ohmuraya, Hyogo College of Medicine.

Prof. Hiroyasu Nakano (left) and Dr. Soh Yamazaki (right)
Key Points:
- In the present study, the research group observed that loss of the transcriptional regulator IκBζ in intestinal epithelial cells in mice led to an imbalance in the intestinal microbiota secondary to reduced antimicrobial activity of the epithelial cells.
- An abnormal increase in numbers of segmental bacteria (SFB), which promote Th17 lymphocyte differentiation in the small intestine, was observed in mice lacking IκBζ in intestinal epithelial cells. These findings highlight symptom exacerbation in a Th17 cell-induced autoimmune disease mouse model
- Reduced antimicrobial activity observed in IκBζ-deficient mice was attributable to reduced release of immunoglobulin-A (an antibody that plays a key role in mucosal immunity) and reduced numbers of Paneth cells that secrete antimicrobial proteins.
- SFB and Th17 cells operate through a regulatory feedback circuit that maintains constant levels of both these components in the intestinal tract of healthy mice. However, a malfunction of this mechanism in mice that lack IκBζ in the intestinal epithelium results in a significant increase in both SFB and Th17 cells, which increases the risk of autoimmune diseases.
Summary:
A healthy gut microbiome is essential for intestinal homeostasis however mechanisms underlying regulation of intestinal bacteria by intestinal epithelial cells remain unclear. In this study, investigation of genetically engineered mice revealed the role of IκBζ, a transcriptional regulator, which regulates the antimicrobial activity of epithelial cells via the interleukin (IL)-17 cytokine primarily released by Th17 cells (lymphocytes that constitute intestinal immune cells). Optimal maintenance of intestinal bacteria via modulation of IκBζ function and IL-17 signaling may serve as potential novel treatment for excessive immune response-mediated diseases.
A healthy gut microbiome is essential for intestinal homeostasis however mechanisms underlying regulation of intestinal bacteria by intestinal epithelial cells remain unclear. In this study, investigation of genetically engineered mice revealed the role of IκBζ, a transcriptional regulator, which regulates the antimicrobial activity of epithelial cells via the interleukin (IL)-17 cytokine primarily released by Th17 cells (lymphocytes that constitute intestinal immune cells). Optimal maintenance of intestinal bacteria via modulation of IκBζ function and IL-17 signaling may serve as potential novel treatment for excessive immune response-mediated diseases.
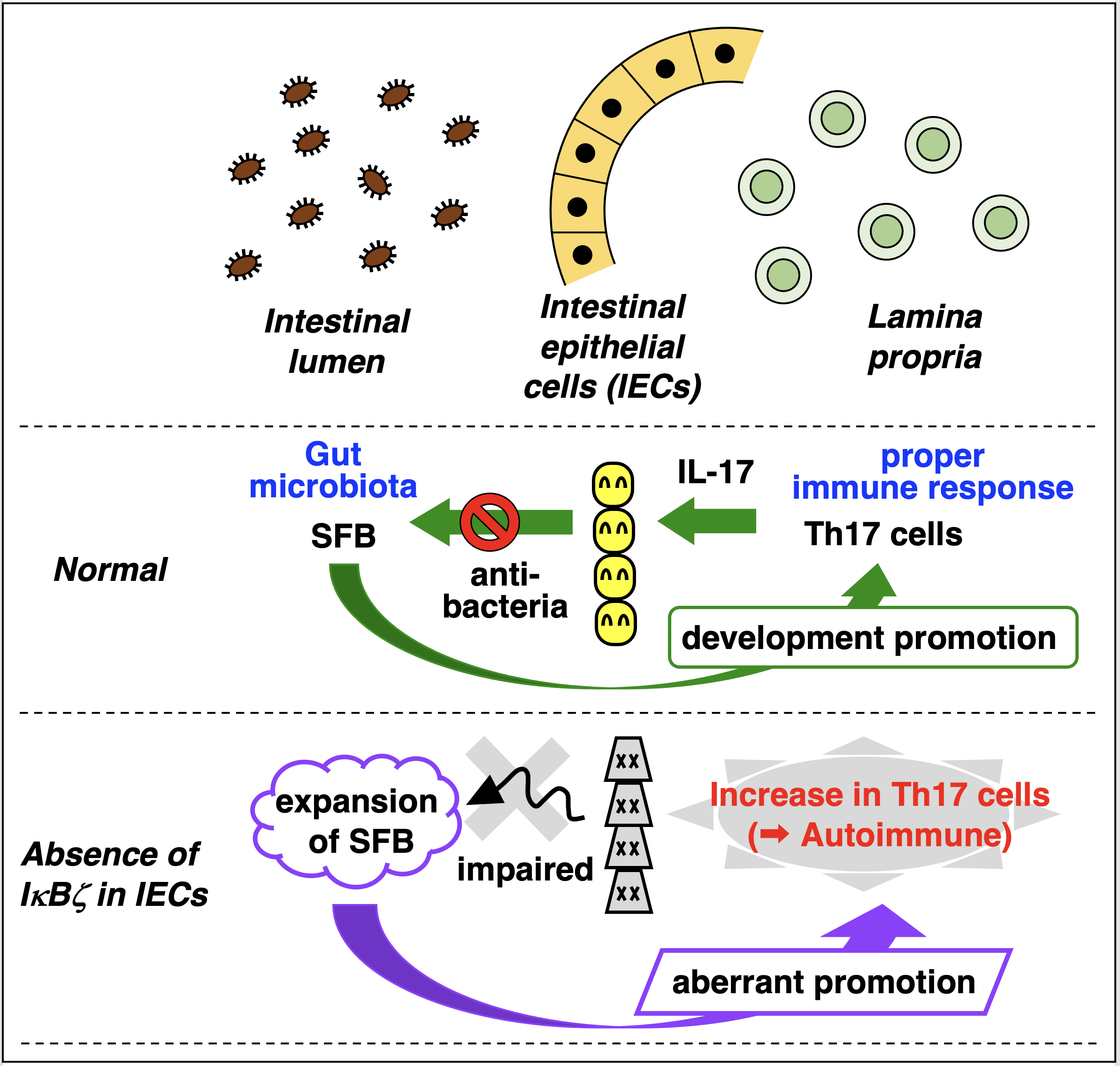
In the normal intestine, IECs control gut bacteria by releasing anti-microbial factors in response to IL-17. Because increase in SFB leads to up-regulation of IL-17 by promoting Th17 cell generation, the numbers of SFB and Th17 cells are maintained constant. In contrast, lack of IκBζ in IECs results in expansion of both SFB and Th17 cells, which can cause the development of autoimmune diseases.
Journal:
Mucosal Immunology August 24 2022 issue
Title
IκBζ controls IL-17-triggered gene expression program in intestinal epithelial cells that restricts colonization of SFB and prevents Th17-associated pathologies.
Authors
Soh Yamazaki*, Naohiro Inohara, Masaki Ohmuraya, Yousuke Tsuneoka, Hideo Yagita, Takaharu Katagiri, Takashi Nishina, Tetuo Mikami, Hiromasa Funato, Kimi Araki, Hiroyasu Nakano*
DOI No.
10.1038/s41385-022-00554-3
Mucosal Immunology August 24 2022 issue
Title
IκBζ controls IL-17-triggered gene expression program in intestinal epithelial cells that restricts colonization of SFB and prevents Th17-associated pathologies.
Authors
Soh Yamazaki*, Naohiro Inohara, Masaki Ohmuraya, Yousuke Tsuneoka, Hideo Yagita, Takaharu Katagiri, Takashi Nishina, Tetuo Mikami, Hiromasa Funato, Kimi Araki, Hiroyasu Nakano*
DOI No.
10.1038/s41385-022-00554-3
READ MORE RESEARCH NEWS - MEDICINE
ACADEMICS
Undergraduate Programs
– Medicine
– Pharmaceutical Sciences
– Science
– Nursing
– Health Science
Graduate Programs
–Medicine
–Pharmaceutical Sciences
–Science
–Nursing
Undergraduate Programs
– Medicine
– Pharmaceutical Sciences
– Science
– Nursing
– Health Science
Graduate Programs
–Medicine
–Pharmaceutical Sciences
–Science
–Nursing
RESEARCH
– News
– Guidelines & Policies
– Support Offices
– Facilities
– Security Export Control
Non-Degree Programs
– Clinical Elective Program
– International Physician Observership Program