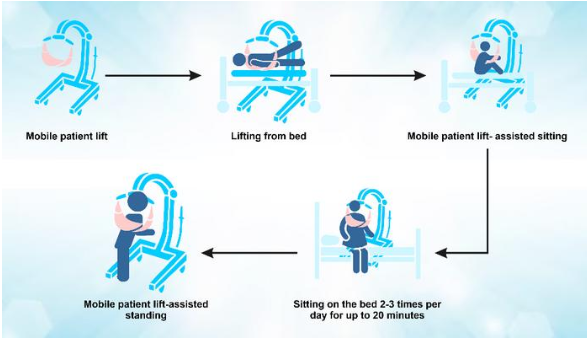
September 7, 2022
Are “sugar chains” the key to cancer immunotherapy?
Novel Glycan Structures and Regulatory Mechanisms Promoting Cancer Cell Death


This is the first study to demonstrate that the Lewis sugar chain added to glycolipids on the surface of cancer cells promotes TRAIL-induced cell death and that the susceptibility of cancer cells to TRAIL-induced death can be predicted by measuring the amount of Lewis sugar chains. This study is expected to aid in the development of new cancer treatment strategies based on the prediction of the therapeutic effects of TRAIL receptor molecular-targeted drugs and cancer immunotherapy.
This research was conducted in collaboration with Professors Eiji Miyoshi and Keiichi Ozono, Osaka University Graduate School of Medicine, Professor Masahiro Inoue, Graduate School of Medicine Kyoto University, Dr. Yasuhide Miyamoto, Osaka International Cancer Center Research Institute, and Dr. Hiroyuki Kaji, National Institute of Advanced Industrial Science and Technology.
Key Points:
- For the first time, the group has shown that specific sugar chains (Lewis sugar chains) added to lipids on cancer cells enhance cancer cell death by a cytokine called TRAIL.
- The study showed that measuring the amount of Lewis glycans on cancer cells or in blood has the potential to predict the therapeutic effect of cancer drugs targeting the TRAIL receptor.
- The TRAIL receptor is expected to be a molecular target for cancer therapy, and many therapeutic agents have been developed. However, some patients do not respond well to treatment owing to the resistance of cancer cells to TRAIL receptor-mediated cell death, and the agents has not yet reached the stage of clinical application.
- TRAIL is a cytokine that plays a role in the tumor immune surveillance mechanism, and this study is expected to enable the prediction of the therapeutic effects of cancer immunotherapy.
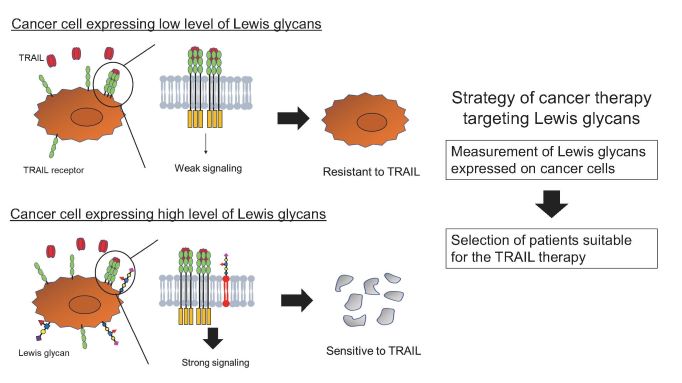
Fig. 1: Lewis glycans promote TRAIL-induced apoptosis
Journal:
Oncogene, August 15, 2022 issue
Title:
Lewis glycosphingolipids as critical determinants of TRAIL sensitivity in cancer cells
Authors:
Tomoya Fukuoka, Kenta Moriwaki*, Shinji Takamatsu, Jumpei Kondo, Miki Tanaka-Okamoto, Azusa Tomioka, Manami Semba, Sachiko Komazawa-Sakon, Yoshihiro Kamada, Hiroyuki Kaji, Yasuhide Miyamoto, Masahiro Inoue, Kazuhiko Bessho, Yoko Miyoshi, Keiichi Ozono, Hiroyasu Nakano, Eiji Miyoshi*
DOI No.
10.1038/s41388-022-02434-3
Oncogene, August 15, 2022 issue
Title:
Lewis glycosphingolipids as critical determinants of TRAIL sensitivity in cancer cells
Authors:
Tomoya Fukuoka, Kenta Moriwaki*, Shinji Takamatsu, Jumpei Kondo, Miki Tanaka-Okamoto, Azusa Tomioka, Manami Semba, Sachiko Komazawa-Sakon, Yoshihiro Kamada, Hiroyuki Kaji, Yasuhide Miyamoto, Masahiro Inoue, Kazuhiko Bessho, Yoko Miyoshi, Keiichi Ozono, Hiroyasu Nakano, Eiji Miyoshi*
DOI No.
10.1038/s41388-022-02434-3
READ MORE RESEARCH NEWS - MEDICINE
ACADEMICS
Undergraduate Programs
– Medicine
– Pharmaceutical Sciences
– Science
– Nursing
– Health Science
Graduate Programs
–Medicine
–Pharmaceutical Sciences
–Science
–Nursing
Undergraduate Programs
– Medicine
– Pharmaceutical Sciences
– Science
– Nursing
– Health Science
Graduate Programs
–Medicine
–Pharmaceutical Sciences
–Science
–Nursing
RESEARCH
– News
– Guidelines & Policies
– Support Offices
– Facilities
– Security Export Control
Non-Degree Programs
– Clinical Elective Program
– International Physician Observership Program