April 21, 2023
Influence of immunosuppressive drugs on the efficacy of novel coronavirus vaccines based on changes in antibody values measured over time following vaccination in patients with rheumatic diseases
A research group that included Dr. Mai Kawazoe and Prof. Toshihiro Nanki of the Division of Rheumatology, Department of Internal Medicine, Toho University Faculty of Medicine, measured values of antibodies to the virus before and up to 6 months after vaccination with the novel coronavirus vaccine in patients with rheumatic diseases, who received immunosuppressive drugs and glucocorticoids and compared these with antibody values in patients with non-rheumatic diseases, who did not receive immunosuppressive drugs. Results showed that serum values of antibodies to the virus were low in patients with rheumatic diseases, and differences were observed in the rate of antibody acquisition and changes in antibody values over time depending on the type of immunosuppressive drug administered. Both symptoms and frequency of adverse reactions were comparable with those in patients with non-rheumatic diseases, which indicates that administration of the novel coronavirus vaccine is safe in patients with rheumatic diseases. Results of this study are expected to lead to higher rates of vaccination coverage and booster vaccination recommendations for novel coronavirus vaccines in the future in patients with rheumatic diseases, which may contribute to reduction in the numbers of patients with novel coronavirus infection and control of severe disease. The results of this study were published on April 10, 2023 in the online edition of the European Journal of Internal Medicine, the official journal of the European Federation of Internal Medicine.
A research group that included Dr. Mai Kawazoe and Prof. Toshihiro Nanki of the Division of Rheumatology, Department of Internal Medicine, Toho University Faculty of Medicine, measured values of antibodies to the virus before and up to 6 months after vaccination with the novel coronavirus vaccine in patients with rheumatic diseases, who received immunosuppressive drugs and glucocorticoids and compared these with antibody values in patients with non-rheumatic diseases, who did not receive immunosuppressive drugs. Results showed that serum values of antibodies to the virus were low in patients with rheumatic diseases, and differences were observed in the rate of antibody acquisition and changes in antibody values over time depending on the type of immunosuppressive drug administered. Both symptoms and frequency of adverse reactions were comparable with those in patients with non-rheumatic diseases, which indicates that administration of the novel coronavirus vaccine is safe in patients with rheumatic diseases. Results of this study are expected to lead to higher rates of vaccination coverage and booster vaccination recommendations for novel coronavirus vaccines in the future in patients with rheumatic diseases, which may contribute to reduction in the numbers of patients with novel coronavirus infection and control of severe disease. The results of this study were published on April 10, 2023 in the online edition of the European Journal of Internal Medicine, the official journal of the European Federation of Internal Medicine.

Key Points:
- In this study, the authors investigated changes over time in the rate of antibody acquisition and antibody values up to 6 months after the administration of two doses of the novel coronavirus vaccine to patients with rheumatic diseases, who received immunosuppressive drugs and glucocorticoid therapy and adhered to the ‘Guidance for COVID-19 Vaccination in Patients With Rheumatic and Musculoskeletal Diseases’ issued by the American College of Rheumatology on withholding drugs.
- Antibody production against the novel coronavirus was higher in non-rheumatic disease group than rheumatic disease group.
- Antibody production was reduced in patients with rheumatic diseases, who received several immunosuppressive drugs (mycophenolate mofetil, azathioprine, abatacept, tumor necrosis factor inhibitors, interleukin-6 inhibitors, and rituximab) and glucocorticoids (specifically, prednisolone >15 mg/day).
- Comparison between patients with rheumatic diseases who received immunosuppressive agents and those with non-rheumatic diseases who did not receive immunosuppressive drugs showed comparable adverse reactions with regard to both symptoms and frequency, which confirms vaccine safety in patients with rheumatic diseases.
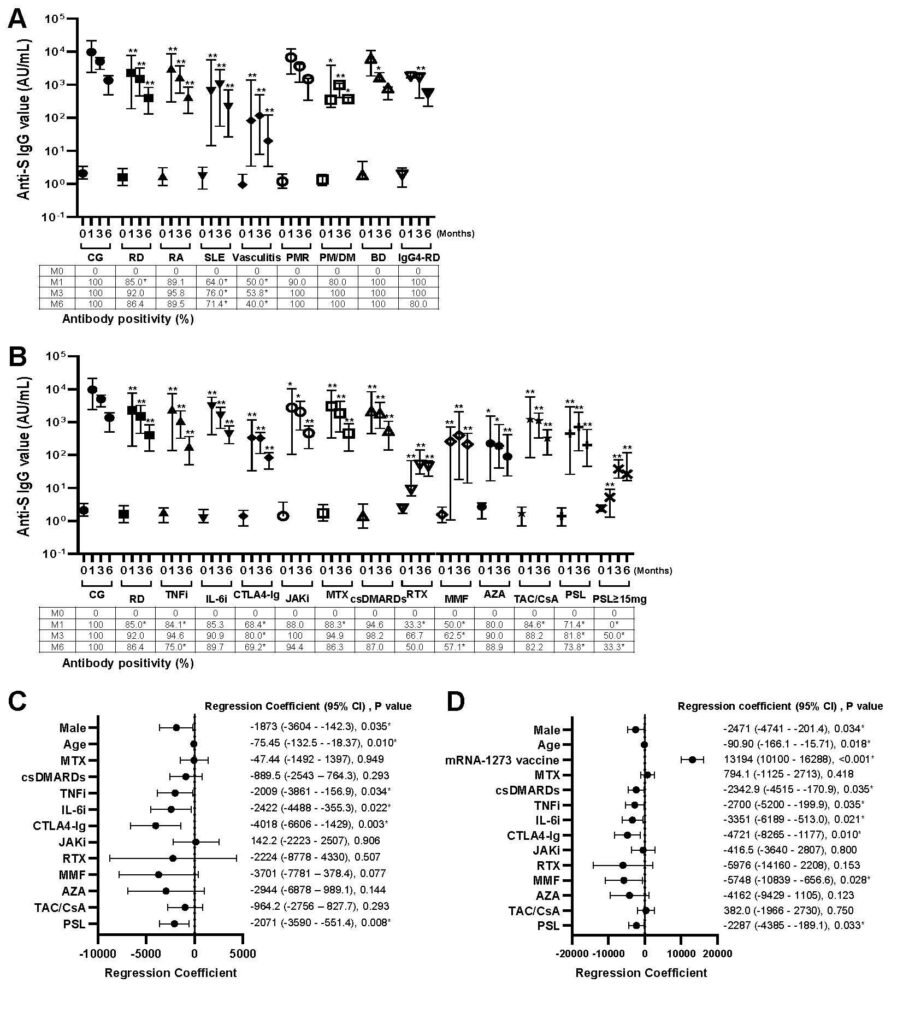
FIgure 1. Effects of the rheumatic diseases and treatments for anti-SARS-CoV-2 spike protein IgG production. (A) Anti-SARS-CoV-2 spike protein IgG values and antibody positivity were measured before and 1, 3, and 6 months after the BNT162b2 mRNA vaccination, indicating as 0, 1, 3 and 6, respectively. Data are expressed as medians (25th to 75th percentiles). *P< 0.05 and **P< 0.01 for comparisons of the RD group and each rheumatic disease with the CG. Data of antibody positivity are expressed as %. *P < 0.05, comparisons between the CG and RD group or each disease. (B) Anti-SARS-CoV-2 spike protein IgG values and antibody positivity. *P< 0.05 and **P< 0.01 for comparisons of the RD group and treatment with the CG. Data of antibody positivity are expressed as %. *P < 0.05, comparisons between the CG and each treatment or RD group. (C) Factors affecting extent of changes in anti-SARS-CoV-2 spike protein IgG values in RD patients receiving the BNT162b2 mRNA, and (D) receiving the BNT162b2 mRNA or mRNA-1273 vaccination. *P< 0.05 by multivariate analysis with a linear mixed model.
Summary:
A few studies have reported high morbidity, hospitalization, and severe illness and mortality rates associated with the novel coronavirus disease (COVID-19) infection, mainly due to immunosuppressive drugs administered to patients with rheumatic diseases. Unvaccinated individuals are at an increased risk of infection; therefore, vaccination is important to prevent infection in such cases. However, the mRNA vaccine that was first available in Japan as a novel coronavirus vaccine (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2] vaccine) was the first product used in clinical practice in humans, and patients who received immunosuppressive drugs were excluded from Phase III clinical trials; therefore, initially, efficacy and safety data were unavailable for patients with rheumatic diseases. Therefore, antibodies against the virus were measured for up to 6 months after SARS-CoV-2 mRNA vaccination to assess the efficacy and safety in patients with rheumatic diseases, who received immunosuppressive drugs. Results showed differences in the rate of antibody acquisition and change in antibody values over time depending on the type of immunosuppressive drug used despite adherence to the immunosuppressive drug withdrawal period recommended by the American College of Rheumatology. Vaccine safety was confirmed in patients with rheumatic diseases based on the observation that adverse reactions with regard to both symptoms and frequency were comparable with those observed in patients with non-rheumatic diseases, who did not receive immunosuppressive drugs. These findings are expected to serve as evidence to improve vaccine coverage in the future in patients with rheumatic diseases and may also encourage vaccine booster doses, particularly in patients administered immunosuppressive drugs, who have difficulty producing antibodies.
A few studies have reported high morbidity, hospitalization, and severe illness and mortality rates associated with the novel coronavirus disease (COVID-19) infection, mainly due to immunosuppressive drugs administered to patients with rheumatic diseases. Unvaccinated individuals are at an increased risk of infection; therefore, vaccination is important to prevent infection in such cases. However, the mRNA vaccine that was first available in Japan as a novel coronavirus vaccine (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2] vaccine) was the first product used in clinical practice in humans, and patients who received immunosuppressive drugs were excluded from Phase III clinical trials; therefore, initially, efficacy and safety data were unavailable for patients with rheumatic diseases. Therefore, antibodies against the virus were measured for up to 6 months after SARS-CoV-2 mRNA vaccination to assess the efficacy and safety in patients with rheumatic diseases, who received immunosuppressive drugs. Results showed differences in the rate of antibody acquisition and change in antibody values over time depending on the type of immunosuppressive drug used despite adherence to the immunosuppressive drug withdrawal period recommended by the American College of Rheumatology. Vaccine safety was confirmed in patients with rheumatic diseases based on the observation that adverse reactions with regard to both symptoms and frequency were comparable with those observed in patients with non-rheumatic diseases, who did not receive immunosuppressive drugs. These findings are expected to serve as evidence to improve vaccine coverage in the future in patients with rheumatic diseases and may also encourage vaccine booster doses, particularly in patients administered immunosuppressive drugs, who have difficulty producing antibodies.
Journal:
European Journal of Internal Medicine April 10, 2023 issue
Title:
Influence of immunosuppressive therapy on longitudinal changes in anti-SARS-CoV-2 spike protein antibodies after two doses of mRNA vaccines in patients with rheumatic diseases
Authors:
Mai Kawazoe, Kotaro Aoki, Wataru Hirose, Shotaro Masuoka, Toshihiro Nanki*
DOI No.
https://doi.org/10.1016/j.ejim.2023.04.008
European Journal of Internal Medicine April 10, 2023 issue
Title:
Influence of immunosuppressive therapy on longitudinal changes in anti-SARS-CoV-2 spike protein antibodies after two doses of mRNA vaccines in patients with rheumatic diseases
Authors:
Mai Kawazoe, Kotaro Aoki, Wataru Hirose, Shotaro Masuoka, Toshihiro Nanki*
DOI No.
https://doi.org/10.1016/j.ejim.2023.04.008
READ MORE RESEARCH NEWS - MEDICINE
ACADEMICS
Undergraduate Programs
– Medicine
– Pharmaceutical Sciences
– Science
– Nursing
– Health Science
Graduate Programs
–Medicine
–Pharmaceutical Sciences
–Science
–Nursing
Undergraduate Programs
– Medicine
– Pharmaceutical Sciences
– Science
– Nursing
– Health Science
Graduate Programs
–Medicine
–Pharmaceutical Sciences
–Science
–Nursing
RESEARCH
– News
– Guidelines & Policies
– Support Offices
– Facilities
– Security Export Control
Non-Degree Programs
– Clinical Elective Program
– International Physician Observership Program