January 7, 2026
Development of a HepaRG Human Hepatocyte Model with Enhanced CYP2D6 Activity
Toward Early Prediction of Drug-Induced Liver Injury and a Promising Alternative to Animal Testing


Key Points
- Establishment of a next-generation hepatocyte model with metabolic capacity comparable to adult human liver tissue
By enhancing CYP2D6 metabolic activity to approximately 8,000-fold relative to conventional HepaRG cells, the team successfully reproduced expression levels comparable to adult human liver tissue in vitro. - Robust model with practical utility, achieved through green fluorescent protein (GFP) visualization and stable functional maintenance
GFP co-expression enabled real-time monitoring of CYP2D6 expression, and high expression levels were maintained even after differentiation into hepatocyte-like cells, allowing detailed tracking of complex drug responses. - Implications for early prediction of drug toxicity and an alternative to animal testing
Experiments with perhexiline, a drug primarily metabolized by CYP2D6, demonstrated improved cell viability in the CYP2D6-enhanced HepaRG cell model. This system facilitates prediction of toxicity associated with inter-individual variability in human CYP2D6 activity, which is difficult to reproduce in animal models, thereby supporting early-stage drug safety assessments and serving as a promising in vitro alternative to animal testing.
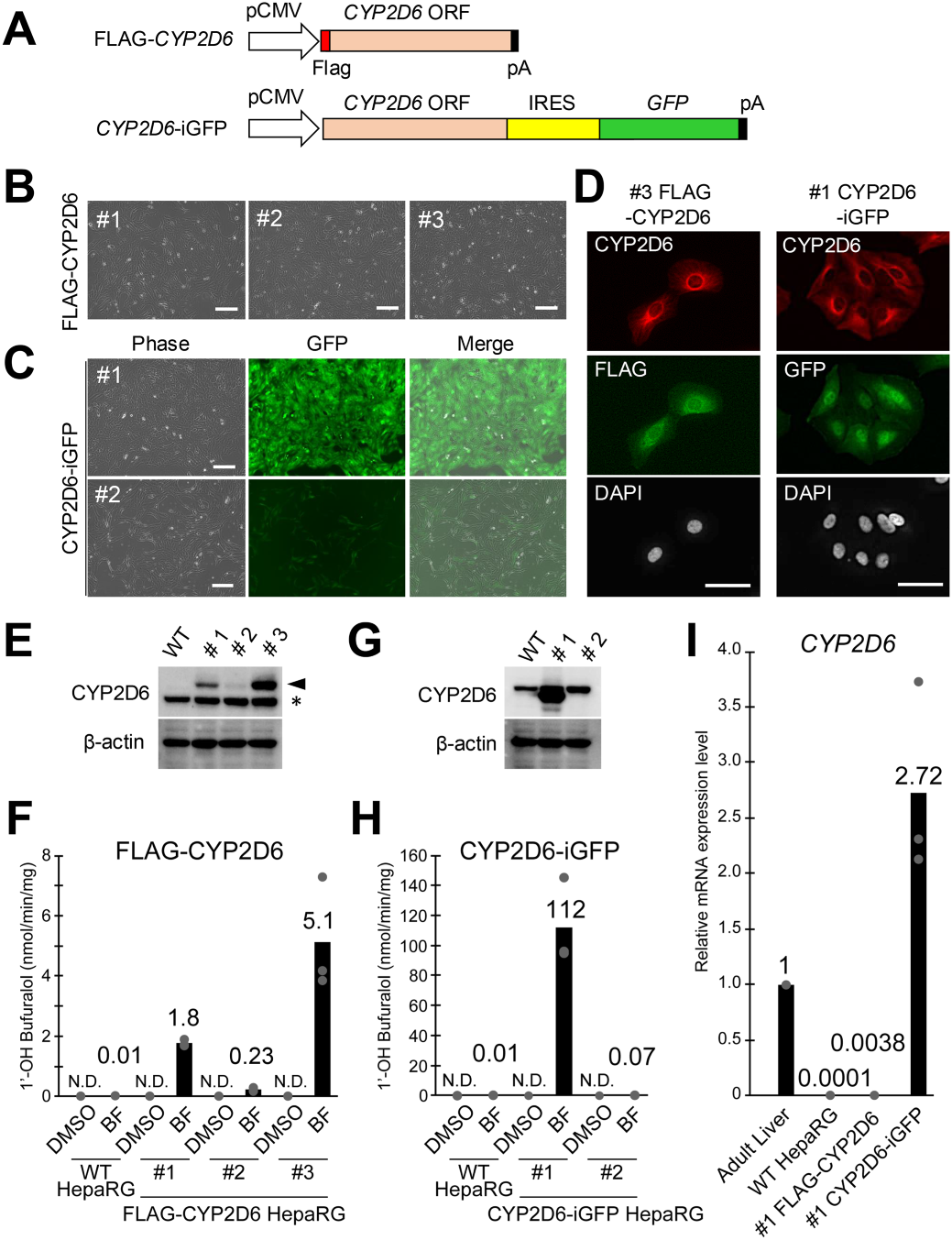
Figure 1: Establishment and characterization of FLAG-CYP2D6 and CYP2D6-iGFP undifferentiated HepaRG cells.
Journal:
PLOS ONE
Title:
Development of a CYP2D6-enhanced HepaRG Cell Model with Improved CYP2D6 Metabolic Capacity
Authors:
Chizuka Obara, Yohei Iizaka, Akari Mine, Yojiro Anzai, Masako Tada*, and Shinpei Yamaguchi*
Published:
December 29, 2025
DOI:
https://doi.org/10.1371/journal.pone.0339559
PLOS ONE
Title:
Development of a CYP2D6-enhanced HepaRG Cell Model with Improved CYP2D6 Metabolic Capacity
Authors:
Chizuka Obara, Yohei Iizaka, Akari Mine, Yojiro Anzai, Masako Tada*, and Shinpei Yamaguchi*
Published:
December 29, 2025
DOI:
https://doi.org/10.1371/journal.pone.0339559
READ MORE RESEARCH NEWS - SCIENCE
ACADEMICS
Undergraduate Programs
– Medicine
– Pharmaceutical Sciences
– Science
– Nursing
– Health Science
Graduate Programs
–Medicine
–Pharmaceutical Sciences
–Science
–Nursing
Undergraduate Programs
– Medicine
– Pharmaceutical Sciences
– Science
– Nursing
– Health Science
Graduate Programs
–Medicine
–Pharmaceutical Sciences
–Science
–Nursing
RESEARCH
– News
– Guidelines & Policies
– Support Offices
– Facilities
– Security Export Control
Non-Degree Programs
– Clinical Elective Program
– International Physician Observership Program