Discovery of a novel mechanism for regulating DNA repair and cell cycle progression
ADP-ribosylation of serine in histone proteins

Dr. Tetsuya Muramoto of Department of Biology, Faculty of Science, Toho University, has published the results of a research collaboration with a research team led by Professor Nicholas D. Lakin at the Oxford University in the UK.
The team has revealed a new finding that ADP-ribosylation, a modification that adds ADP to serine in “histone proteins, which functions like a spool to store DNA in the nucleus”, is involved in regulating DNA repair and cell cycle progression. This research result is expected to provide an in-depth illumination on the mechanism of cancer development and DNA repair pathways.
This research was published in the British scientific journal “Nature Communications” on January 13, 2022.

The team has revealed a new finding that ADP-ribosylation, a modification that adds ADP to serine in “histone proteins, which functions like a spool to store DNA in the nucleus”, is involved in regulating DNA repair and cell cycle progression. This research result is expected to provide an in-depth illumination on the mechanism of cancer development and DNA repair pathways.
This research was published in the British scientific journal “Nature Communications” on January 13, 2022.

- It was shown that the serine ADP-ribosylation modification of histone proteins induced by DNA damage maintains genome stability by regulating DNA repair and cell cycle progression.
- By manipulating histone genes, they succeeded in separately analyzing the roles of “two types of histone modifications”: phosphorylation of serine that occurs during mitosis, and ADP-ribosylation of serine that occurs during DNA damage.
- The findings are expected to be useful for understanding the fundamental mechanisms; that control “DNA repair and genome stability”, and of cancer development too.
“Poly (ADP-ribose) polymerase (PARP)” adds ADP-ribose moieties to target proteins. ADP-ribosylation that occurs in response to DNA damage, is involved in the fundamental mechanism of maintaining genome stability through DNA repair. Compounds that inhibit PARP are used as anticancer agents because they induce apoptosis by interfering with the DNA repair pathway necessary for cancer cell proliferation. Against this background, research on the mechanism of ADP-ribosylation, which regulates DNA repair, has been of interest globally. However, it has remained unclear how the ADP-ribosylation of serine in histone protein is involved in the mechanisms regulating DNA repair and genome stability. One of the main reasons for this is that there was no experimental system to specifically analyze serine ADP-ribosylation induced by DNA damage. Therefore, the research team succeeded in specifically analyzing the function of serine ADP-ribosylation, which had been difficult until now, by modifying the histone gene of a model organism, “cellular slime mold”. The results showed that the serine ADP-ribosylation modification regulates DNA repair and cell cycle progression. This achievement is expected to clarify the fundamental mechanisms that regulate “DNA repair and genome stability”, and instigate the development of new drugs.

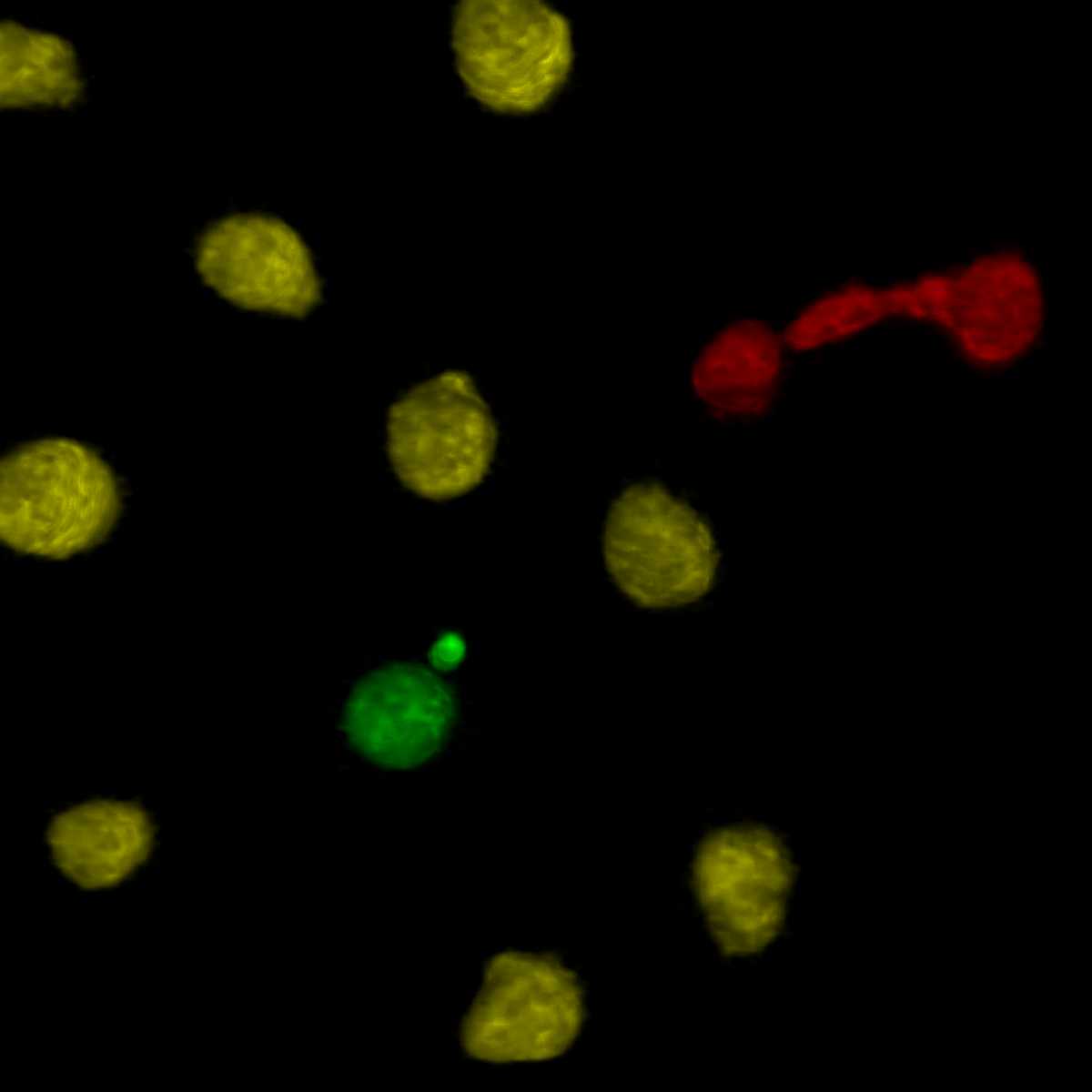
The mutant exhibits difficulties completing cytokinesis, resulting in
anaphase bridge (red) and abnormal micronucleus (green).
“Poly (ADP-ribose) polymerase (PARP)” adds ADP-ribose moieties to target proteins. ADP-ribosylation that occurs in response to DNA damage, is involved in the fundamental mechanism of maintaining genome stability through DNA repair. Compounds that inhibit PARP are used as anticancer agents because they induce apoptosis by interfering with the DNA repair pathway necessary for cancer cell proliferation. Against this background, research on the mechanism of ADP-ribosylation, which regulates DNA repair, has been of interest globally. However, it has remained unclear how the ADP-ribosylation of serine in histone protein is involved in the mechanisms regulating DNA repair and genome stability. One of the main reasons for this is that there was no experimental system to specifically analyze serine ADP-ribosylation induced by DNA damage. Therefore, the research team succeeded in specifically analyzing the function of serine ADP-ribosylation, which had been difficult until now, by modifying the histone gene of a model organism, “cellular slime mold”. The results showed that the serine ADP-ribosylation modification regulates DNA repair and cell cycle progression. This achievement is expected to clarify the fundamental mechanisms that regulate “DNA repair and genome stability”, and instigate the development of new drugs.
Linking DNA repair and cell cycle progression through serine ADP-ribosylation of histones
Authors:Julien Brustel, Tetsuya Muramoto, Kazuki Fumimoto, Jessica Ellins, Catherine J. Pears & Nicholas D. Lakin
DOI Number:10.1038/s41467-021-27867-4
READ MORE RESEARCH NEWS - SCIENCE
Undergraduate Programs
– Medicine
– Pharmaceutical Sciences
– Science
– Nursing
– Health Science
Graduate Programs
–Medicine
–Pharmaceutical Sciences
–Science
–Nursing
RESEARCH
– News
– Guidelines & Policies
– Support Offices
– Facilities
– Security Export Control
Non-Degree Programs
– Clinical Elective Program
– International Physician Observership Program